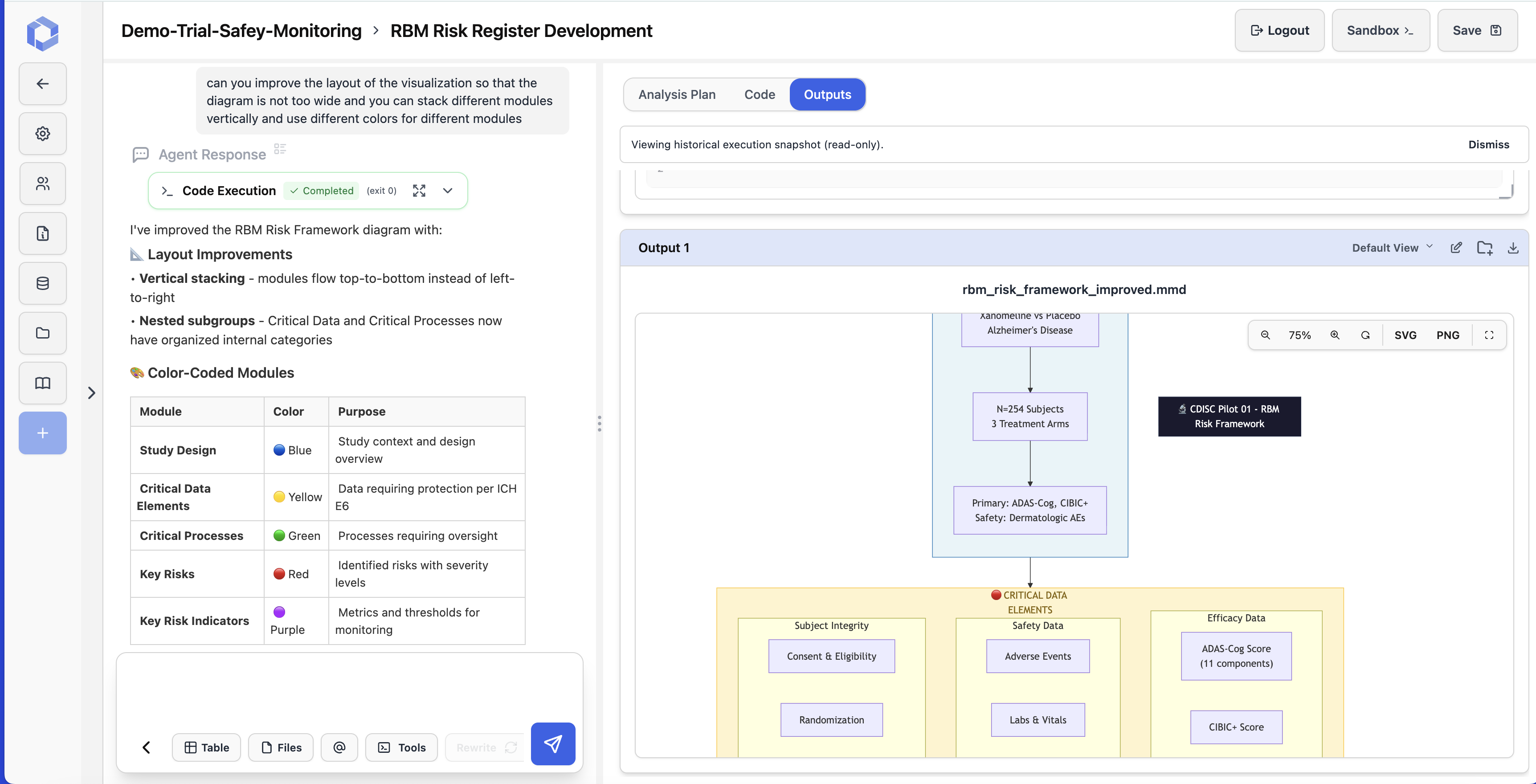
RBM Risk Framework Development
AI develops a comprehensive Risk-Based Monitoring framework with color-coded modules: Study Design (blue), Critical Data Elements (yellow), Critical Processes (green), Key Risks (red), and Key Risk Indicators (purple) per ICH E6(R2) guidelines.
Key Capabilities
RBM risk framework development per ICH E6(R2)
Risk identification and prioritization by severity
Monitoring plan and governance structure generation
Site risk scoring and heatmap visualization
Targeted site visit agenda generation
Data quality and consistency checks
Lab value distribution and outlier detection
Outlier patient identification and flagging
Automatic query generation and recommendations
Key Risk Indicator (KRI) configuration and tracking
Typical Users
Ready to Transform Your Workflow?
See how TrialMind can accelerate your clinical trial operations with AI-powered automation.
Trusted by Leading Organizations
From top pharma companies to academic medical centers









