1
Adverse Events Severity Analysis
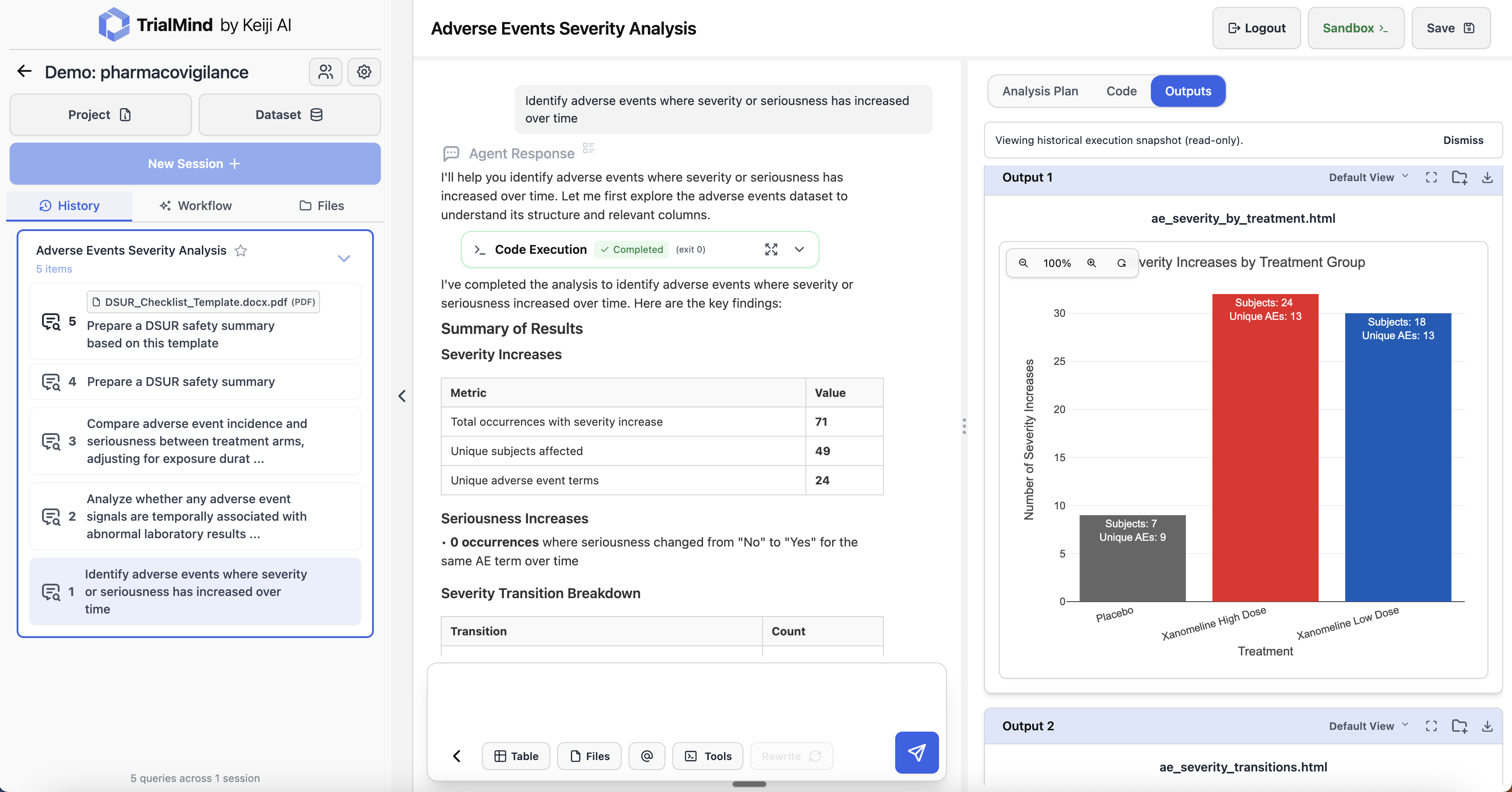
Identify adverse events where severity or seriousness has increased over time. Visualize severity trends by treatment group to detect emerging safety concerns.
Features
Key Capabilities
Automated adverse event coding and classification
Signal detection using statistical and ML methods
Causality assessment support
Aggregate safety reporting and trend analysis
SUSAR identification and expedited reporting
Benefit-risk assessment dashboards
Typical Users
Pharmacovigilance ScientistsSafety PhysiciansDrug Safety AssociatesMedical Monitors
Ready to Transform Your Workflow?
See how TrialMind can accelerate your clinical trial operations with AI-powered automation.
Trusted Partners
Trusted by Leading Organizations
From top pharma companies to academic medical centers









