1
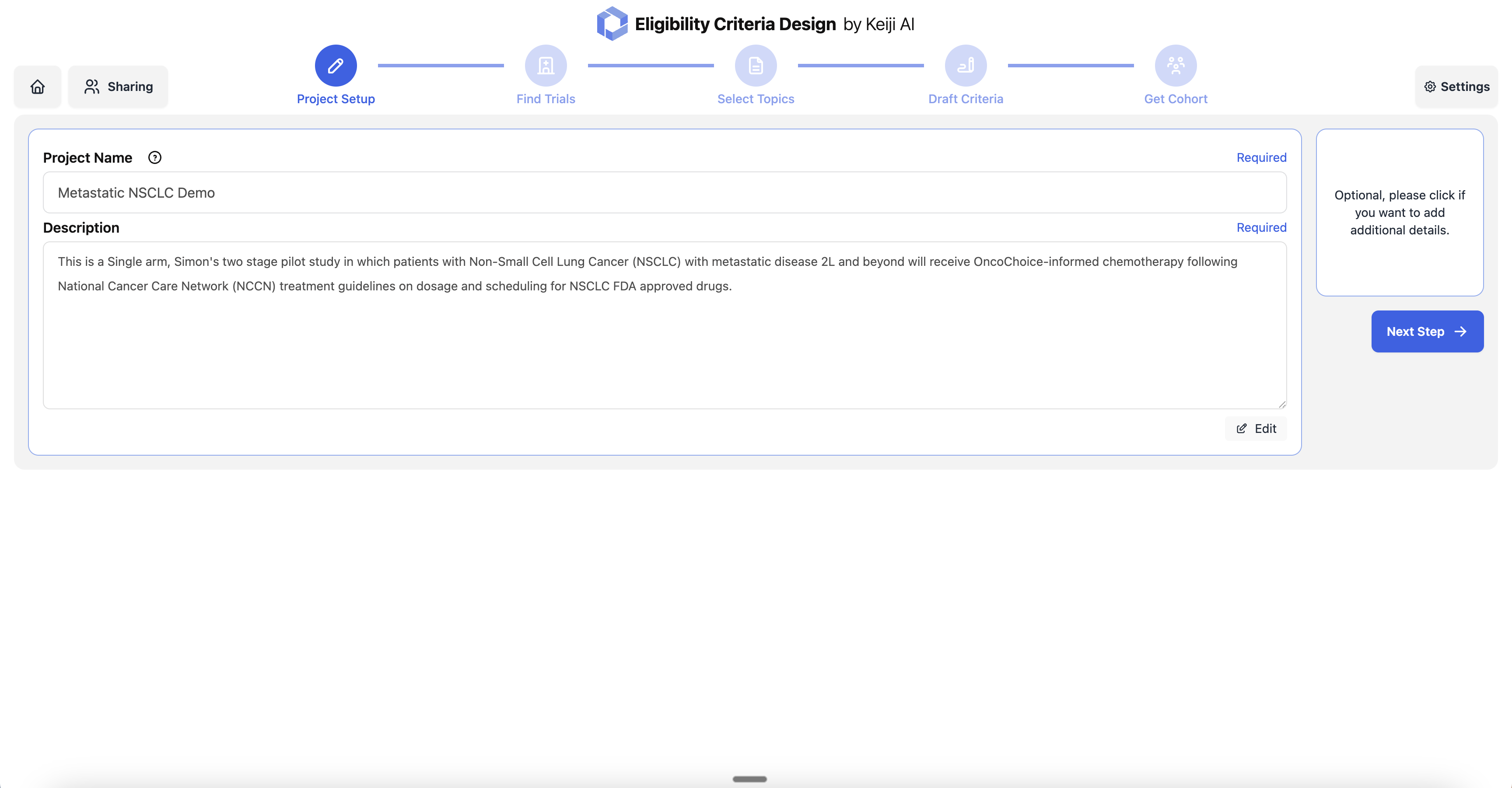
Project Setup
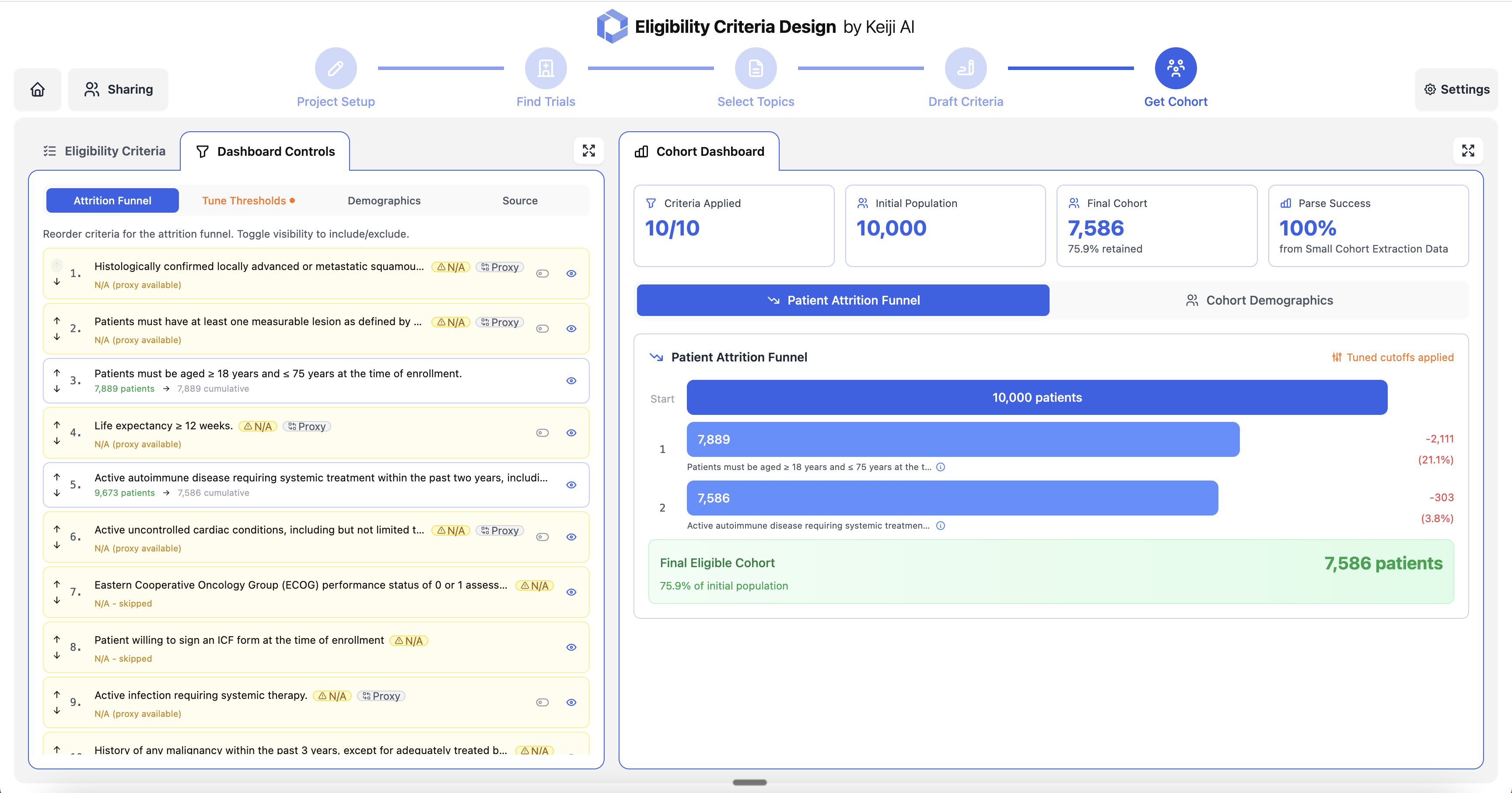
Start by defining your clinical trial project with a name and detailed description. Specify the study design, target population, and therapeutic approach to guide the eligibility criteria generation process.
Features
Key Capabilities
Protocol synopsis generation from study objectives
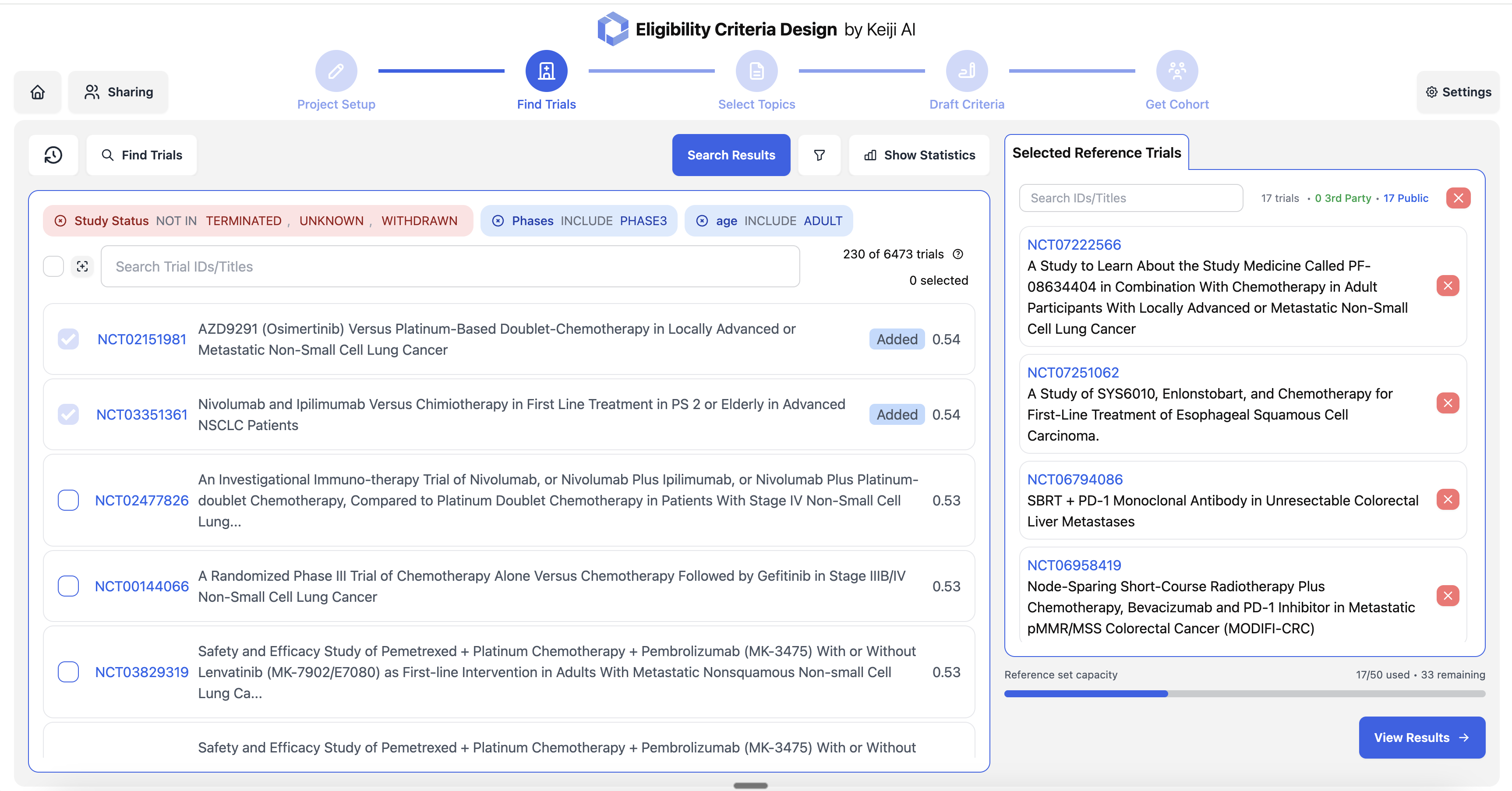
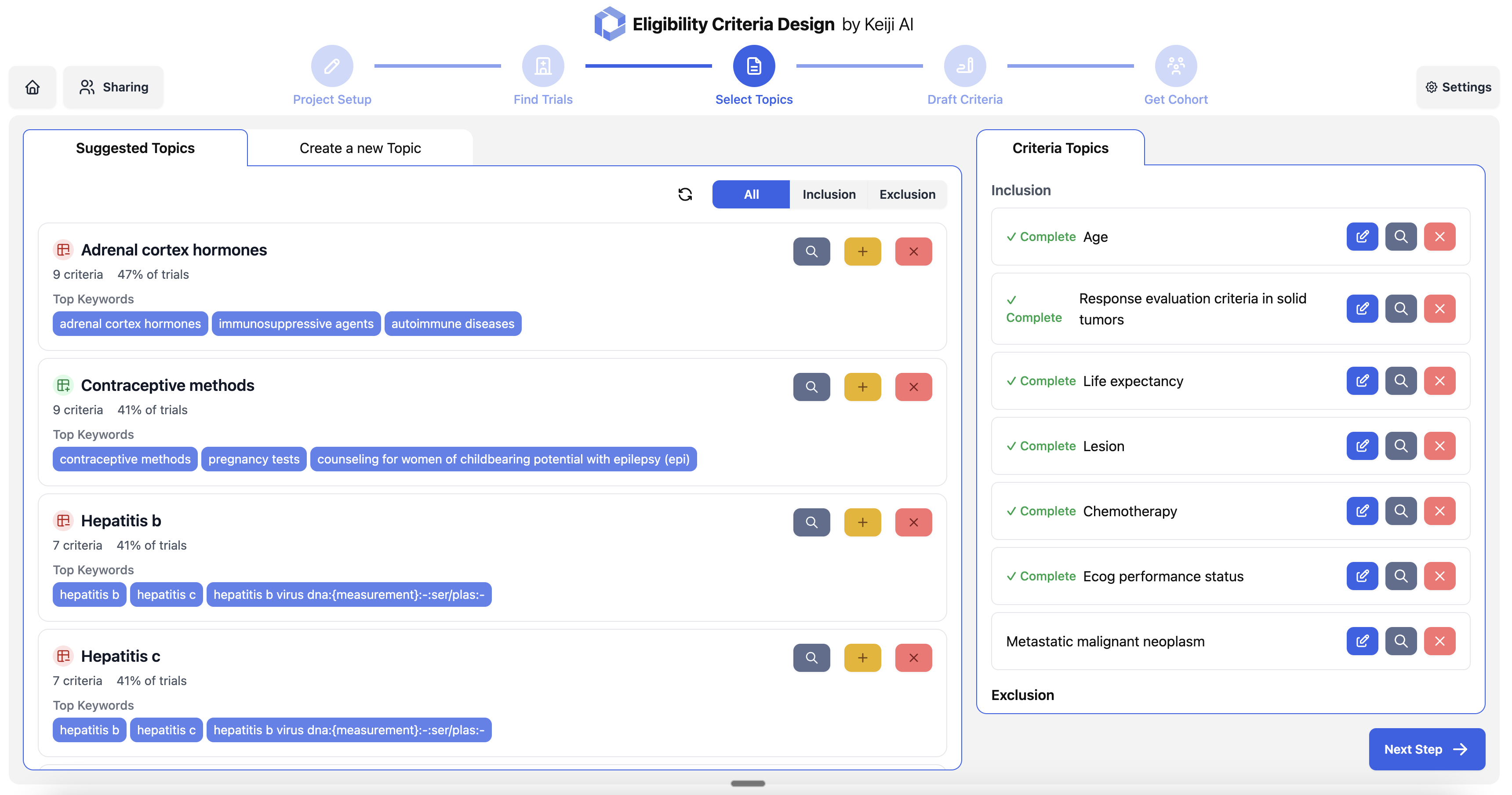
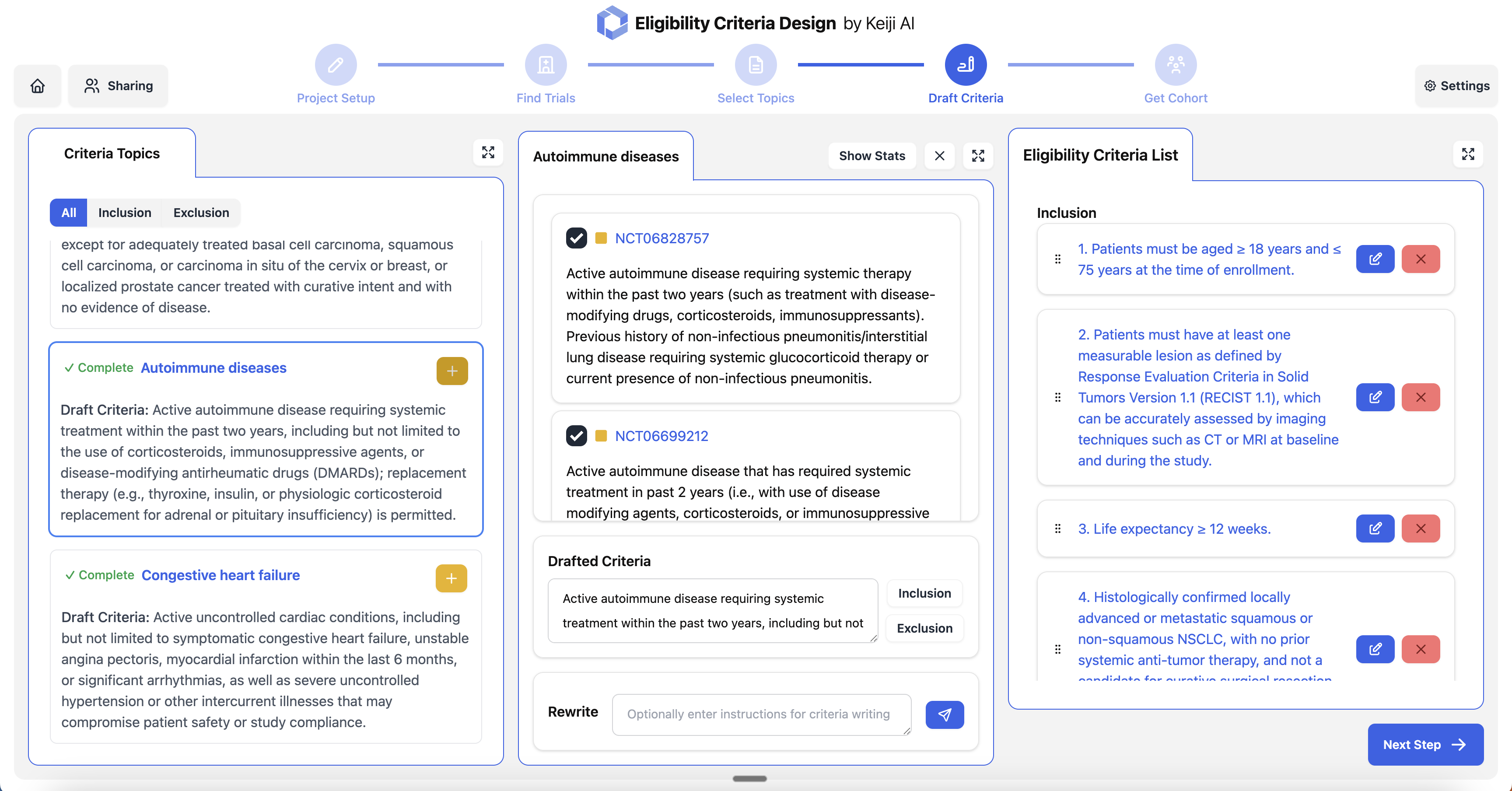
Eligibility criteria optimization based on historical data
Endpoint selection informed by regulatory precedents
Sample size estimation with adaptive design support
Competitive trial landscape analysis
Risk assessment and mitigation planning
Typical Users
Clinical ScientistsMedical DirectorsRegulatory AffairsBiostatisticians
Ready to Transform Your Workflow?
See how TrialMind can accelerate your clinical trial operations with AI-powered automation.
Trusted Partners
Trusted by Leading Organizations
From top pharma companies to academic medical centers









